The farmer felt the tick bite while herding sheep in rural Turkey.
He removed it without much concern—tick bites were common in his work. Three days later, fever hit. Then the bleeding started. From his gums. From under his skin. From places that shouldn’t bleed.
By the time he reached the hospital, doctors knew immediately: Crimean-Congo hemorrhagic fever (CCHF). One of the deadliest tick-borne diseases in the world.
Despite intensive care, he died eight days after that tick bite. He was 42 years old.
CCHF kills approximately 30% of people it infects. It has no approved specific treatment. No vaccine exists for humans. And it’s spreading to new regions as climate change expands tick habitats and livestock movement increases contact between humans, animals, and infected ticks.
This is one of the most dangerous viral hemorrhagic fevers most people have never heard of.
A Disease with Two Names from Two Continents
The disease was first identified in the Crimean Peninsula in 1944 among Soviet soldiers. Doctors called it Crimean hemorrhagic fever.
Twenty-five years later in 1969, scientists made a startling discovery: a disease identified in 1956 in the Congo Basin was caused by the identical virus. The pathogen killing people in the Congo was the same one that had struck down soldiers in Crimea.
The linkage of two place names—one in Eastern Europe, one in Central Africa—created the current name: Crimean-Congo hemorrhagic fever.
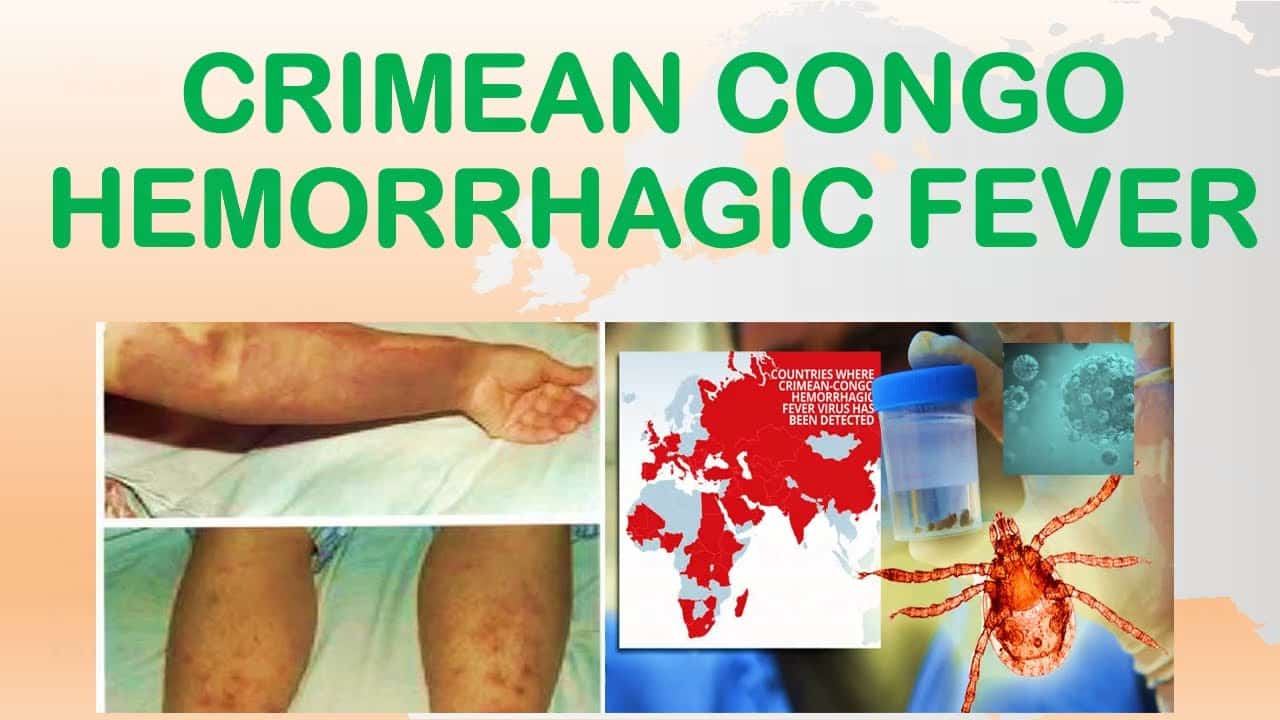
The geographic separation reveals something important: this isn’t a localized threat. CCHF is endemic across vast regions spanning Africa, the Balkans, the Middle East, and Asia. Millions of people live in areas where infected ticks exist.
Dr. Hassan works in infectious disease surveillance in the Eastern Mediterranean. He explained the scope: “CCHF is everywhere ticks and livestock intersect with human populations. Every spring and summer, we watch for outbreaks. Every slaughter season, we hold our breath. The virus is out there constantly, waiting for the next human contact.”
How a Tick Bite Turns Deadly
CCHF spreads primarily through ticks—specifically Hyalomin ticks that feed on livestock and wild animals. When an infected tick bites a human, it can transmit the virus.
But tick bites aren’t the only route.
The virus also spreads through contact with blood or tissues from infected animals, particularly during and immediately after slaughter. Farmers, herders, slaughterhouse workers, and veterinarians face enormous risk when handling infected livestock.
Hospital transmission can occur too. Healthcare workers have contracted CCHF from infected patients’ blood and body fluids when infection control measures fail.
The incubation period varies by transmission route. After a tick bite, symptoms typically appear in 1-3 days (maximum 9 days). After contact with infected blood or tissues, the incubation period is usually 5-6 days (maximum 13 days).
This variability complicates surveillance. By the time someone develops symptoms, they may not remember the tick bite. They may not connect their illness to livestock contact days earlier.
The Devastating Progression
Symptom onset is sudden and frightening.
Fever strikes first, along with severe muscle aches, dizziness, neck pain, backache, and headaches. Eyes become sore and sensitive to light. Many patients experience nausea, vomiting, diarrhea, abdominal pain, and sore throat in the early stages.
Then the mood changes begin. Patients become agitated, confused, and experience sharp emotional swings.
After 2-4 days, the agitation often gives way to sleepiness, depression, and extreme fatigue. Abdominal pain localizes to the upper right side as the liver begins swelling—a sign of hepatitis developing.
The heart races. Lymph nodes swell. Then comes the hallmark sign that gives hemorrhagic fevers their name: bleeding.
Small red spots called petechiae appear on the skin and inside the mouth and throat. These are areas where blood vessels have broken and bled into surrounding tissue. The petechiae can progress to larger bruise-like patches called ecchymoses.
Internal bleeding follows. Severely ill patients experience rapid kidney deterioration, sudden liver failure, or pulmonary failure after the fifth day of illness.
Maria, a veterinarian in Bulgaria, survived CCHF after treating an infected cow. She described the experience: “The bleeding was terrifying. My gums bled constantly. I could see hemorrhages spreading under my skin like dark clouds. I was convinced I was dying. The doctors told me later my liver was failing. I was one of the lucky ones.”
No Approved Treatment Exists
Here’s the brutal truth: there is currently no specific antiviral treatment approved for Crimean-Congo hemorrhagic fever.
When patients arrive at hospitals, doctors can only provide intensive supportive care. They manage fluids carefully, treat specific symptoms as they arise, support failing organs, and hope the patient’s immune system can fight off the virus before it causes irreversible damage.
This supportive care does improve survival chances. Patients who receive early, aggressive medical intervention have better outcomes than those who reach care late.
But with a 30% mortality rate, odds remain grim. Death typically occurs in the second week of illness.
For those who survive, recovery is slow. Improvement generally begins around day 9 or 10 after symptom onset, but complete recovery takes weeks or months.
The lack of specific treatment makes CCHF particularly frightening for healthcare workers. They face infection risk from patients while having no effective medicine to offer if they contract the virus themselves.
The Prevention Challenge
Preventing CCHF is extraordinarily difficult.
The tick-animal-tick transmission cycle operates continuously in nature, usually undetected. Infected animals—cattle, sheep, goats, wild animals—show no symptoms. The infection is invisible until it jumps to humans.
Ticks are numerous and widespread across endemic regions. Tick control using acaricides (tick-killing chemicals) is only realistic for well-managed commercial livestock operations. Small farmers and herders—the populations at highest risk—rarely have resources for comprehensive tick control.
No vaccine exists for human use. No vaccine is widely available for livestock either.
In the absence of medical interventions, prevention relies entirely on reducing exposure through education and behavior change.
Dr. Yilmaz works on CCHF prevention programs in Turkey. He outlined the approach: “We teach livestock workers to wear protective clothing—long sleeves, long pants, gloves when handling animals. We educate about tick checks and safe tick removal. We emphasize that slaughter must happen in controlled settings with protective equipment, not in homes or fields. We train healthcare workers in strict infection control.”
These measures work when implemented consistently. But compliance is challenging, especially in rural communities where traditional practices persist and protective equipment may be expensive or unavailable.
The Climate Change Connection
Climate change is altering CCHF risk patterns.
Rising temperatures allow ticks to expand their ranges northward and to higher elevations. Regions that were previously too cold for Hyalomin ticks now provide suitable habitat.
Changing livestock movement patterns driven by climate variability, land use changes, and economic factors bring animals and their ticks into new areas and closer to human populations.
Increased international livestock trade spreads infected ticks across borders faster than surveillance systems can track them.
Recent outbreaks have occurred in previously unaffected regions, suggesting the endemic zone is expanding.
What Must Happen
CCHF demands urgent attention despite receiving relatively little compared to other infectious diseases.
Vaccine development must accelerate. Several candidate vaccines are in research pipelines, but funding and clinical trials move slowly for diseases that primarily affect poor rural populations in developing countries.
Antiviral drug development needs investment. Ribavirin has been used off-label with unclear benefit, but no proven specific therapy exists.
Surveillance systems require strengthening. Early detection of outbreaks enables rapid response, patient isolation, and contact tracing before the virus spreads widely.
Healthcare worker training in infection prevention and control must be universal in endemic regions. Nosocomial transmission is entirely preventable with proper protocols and equipment.
Public education campaigns must reach at-risk populations with practical information in formats they can understand and implement.
The Bottom Line
Crimean-Congo hemorrhagic fever represents everything dangerous about emerging infectious diseases: high mortality, no specific treatment, no vaccine, expanding geographic range, and transmission patterns that bring humans into repeated contact with infected animals and ticks.
Every spring and summer, the risk resurges as tick populations peak. Every slaughter season, livestock workers face danger. Every CCHF patient threatens healthcare workers.
The disease will remain a serious threat until vaccines and treatments become available and until livestock management practices change to reduce human exposure.
Back to that farmer in Turkey who died from a single tick bite. His death was documented, studied, and added to surveillance data. His family received counseling about prevention. But he’s gone, killed by a virus we still can’t effectively treat.
How many more will die before we develop the tools to fight back?
For more information:
Observer Voice is the one stop site for
National,
International
news,
Sports,
Editor’s Choice,
Art/culture contents,
Quotes and much more. We also cover
historical contents. Historical contents includes
World History,
Indian History, and what happened
today. The website also covers
Entertainment across the India and World.
Follow Us on
Twitter,
Instagram,
Facebook,
& LinkedIn