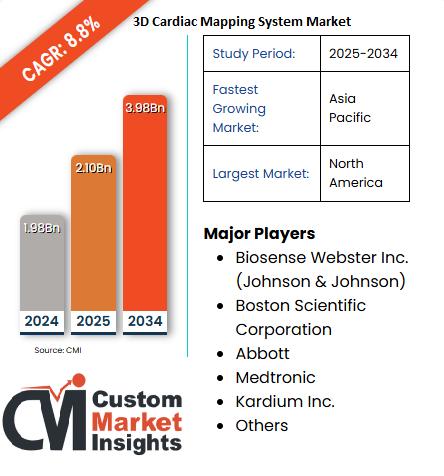
More Than 50,000 People Have Benefited From This Treatment
After its successful clinical application in 2010 on two terminally ill leukemia patients, the first CAR-T cell therapy was approved by the U.S. Food and Drug Administration (FDA) in 2017 for use in children and young adults with refractory acute leukemias and certain refractory lymphomas. One year later, the European Medicines Agency (EMA) also authorized its use across the European Union. To date, more than 50,000 patients with blood cancers have been successfully treated. “Today it is widely accepted that genetically engineered immunity, exemplified by CAR-T cells, can succeed against these types of cancer where no other treatment, chemotherapy, or bone marrow transplantation had previously done so,” Sadelain emphasizes.
So far, CAR-T cells have not achieved the same level of success in solid tumors, such as breast, colon, pancreatic, or lung cancers, as they have in patients with blood cancers. This is the next major challenge in genetically engineered immunotherapy—and one both award-winning scientists are actively pursuing.
“If the same approach is applied to solid tumors, CAR-T cells do not work as effectively as they do in leukemia and lymphoma,” Sadelain acknowledges. Both researchers, however, are confident it’s only a matter of time: “Thousands of laboratories around the world, including my own, are working to make CAR-T cells effective in solid tumors, and I am convinced that we will succeed,” June affirms.